CRISPR-Cas9 (N-Term) Antikörper
-
- Target
- CRISPR-Cas9
-
Bindungsspezifität
- N-Term
-
Reaktivität
- Streptococcus pyogenes
-
Wirt
- Maus
-
Klonalität
- Monoklonal
-
Konjugat
- Unkonjugiert
-
Applikation
- Western Blotting (WB), Immunofluorescence (IF), Immunoprecipitation (IP), Immunocytochemistry (ICC)
- Spezifität
- This antibody should recognize Cas9 and dCas9 based on the antigen design
- Aufreinigung
- Protein A
- Immunogen
- This antibody was raised against a recombinant protein within the N-terminal region of Streptococcus pyogene Cas9
- Klon
- 7A9-3A3
- Isotyp
- IgG1 kappa
-
-
- Applikationshinweise
- Optimal antibody dilution should be determined by titration, however as a guideline try at,IB 0.1-1 μg/mL
- Kommentare
-
Myeloma, fusion partners: X63.Ag8-653
- Beschränkungen
- Nur für Forschungszwecke einsetzbar
-
- by
- Group Kleinlogel, Department of Physiology, University of Bern, Bern, Switzerland
- No.
- #104239
- Datum
- 28.05.2020
- Antigen
- SpCas9
- Chargennummer
- 1060814
- Validierte Anwendung
- Western Blotting
- Positivkontrolle
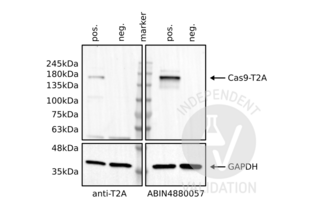
HEK293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP
- Negativkontrolle
Non-transfected HEK293 cells
- Bewertung
Passed. ABIN4880057 specifically recognizes the Cas9 protein in HEK293 cells.
- Primärantikörper
- ABIN4880057
- Sekundärantikörper
- HRP-conjugated goat anti-mouse antibodies (Jackson Immuno Research, 115-035-146)
- Full Protocol
- Grow human embryonic kidney (HEK293) cells (ECACC, 85120602) in DMEM (Sigma, D5671, lot RNB68272) supplemented with 10% Fetal calf Serum (Seraglob, S70500, lot 208/203142), L-Alanyl-L-Glutammine (Merck, Cat. K0302), and Penicillin-Streptomycin (Sigma, P4333, lot 058M4857V) at 37 °C and 5% CO2.
- Plate 0.5x106 cells/ml in 2 ml/well of cells in a 6 well plate.
- Grow cells for 24 h at 37 °C and 5% CO2.
- Transfect cells with 2 µg/well of a plasmid expressing human codon optimized SpCas9-T2A-eGFP under the control of the ubiquitous promoter CMV using the calcium phosphate method. For each well, prepare 150 μl 0.25 M CaCl2, add DNA, add 150 μl HBS, incubate for 3min at RT, and add the whole mix to the cells.
- Change the media after 4-6 h.
- Grow cells for 72 h at 37 °C and 5% CO2.
- Harvest cells with PBS and lyse them on ice for 30min with 50µl/well of RIPA buffer (25 mM TrisHCl pH7-8, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100 or NP-40).
- Complete the lysis with a freeze/taw cycle.
- Determine total protein content of the lysates using Pierce BCA protein assay (Thermo Fisher, 23221).
- Denature 25 μg of total protein for 5min at 95 °C in 20 μl Laemmli SDS sample buffer and subsequently separate them on a denaturing 4-20% Mini-PROTEAN TGX Stain-Free Gel (Bio-Rad, 456-8094) for 20 min at 100 V and 2 h at 130 V.
- Transfer proteins onto Immobilion-P transfer membrane (Immobilion, IPVH00010) with a Western blotting system for 75 min at 100 V.
- Block the membrane with TBST 5% milk for 1 h at RT.
- Incubate with primary
- mouse anti-cas9 antibody (antibodies-online, ABIN4880057, batch n: 1060814) diluted 1:1,000 in TBST 5% milk overnight at 4 °C.
- mouse anti-2A peptide antibody (antibodies-online, ABIN5074774, lot A-12) diluted 1:1,000 in TBST 5% milk overnight at 4 °C.
- mouse anti-GAPDH antibody (Fitzgerald, 10R-G09a, lot 2417) diluted 1:40,000 in TBST 5% milk overnight at 4 °C.
- Wash membrane 3x for 5 min with TBST buffer.
- Incubate with secondary HRP-conjugated goat anti-mouse antibodies (Jackson Immuno Research, 115-035-146) diluted 1:3,000 in TBST 5% milk for 1 h at RT.
- Wash membrane 3x for 10 min with TBST buffer.
- Reveal protein bands on a ChemiDoc MP imaging system (Bio-Rad, 17001402) using Westar Sun ECL Substrate (Cyanagen, XLS063).
- Anmerkungen
The insertion of a 2A peptide between 2 coding sequences leads to the translation of a unique protein which is then spliced in two proteins by the cell machinery. In this process, the 2A peptide stays bound to the C-terminus of the first protein and it can be used as a tag. In this experiment, the T2A peptide is bound to the Cas9 protein. As a consequence, the use of the T2A peptide or the Cas9 antibody should detect a band at the same molecular weight.
The Cas9 antibody ABIN4880057 reveals a protein of the expected molecular weight of antigen in lysates of HEK293 cells. The protein bands are only visible in the positives but not the negative controls. Importantly, in the Cas9 blot, the intensity of the band appears stronger than in the T2A-peptide blot suggesting that the Cas9 antibody is stronger than the T2A peptide antibody.
Validierung #104239 (Western Blotting)![Erfolgreich validiert 'Independent Validation' Siegel]()
![Erfolgreich validiert 'Independent Validation' Siegel]() Validierungsbilder
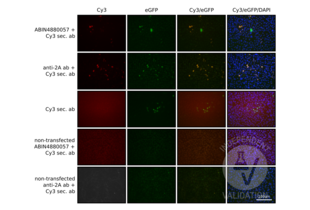
Validierungsbilder![HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.]() HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.
Protokoll
HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.
Protokoll -
- by
- Group Kleinlogel, Department of Physiology, University of Bern, Bern, Switzerland
- No.
- #104240
- Datum
- 03.07.2020
- Antigen
- SpCas9
- Chargennummer
- 1060814
- Validierte Anwendung
- Immunofluorescence
- Positivkontrolle
HEK cells transfected with a plasmid expressing SaCas9-T2A-eGFP
anti-T2A antibody
- Negativkontrolle
Non-transfected HEK293 cells
- Bewertung
Passed. ABIN4880057 specifically recognizes SaCas9 in HEK cells expressing SaCas9-T2A-eGFP.
- Primärantikörper
- ABIN4880057
- Sekundärantikörper
- goat anti-mouse Cy3 (Invitrogen, A10521)
- Full Protocol
- Grow human embryonic kidney HEK293 cells (ECACC, 85120602) in DMEM (Sigma, D5671, lot RNB68272) supplemented with 10% Fetal calf Serum (Seraglob, S70500, lot 208/203142), L-Alanyl-L-Glutammine (Merck, Cat. K0302), and Penicillin-Streptomycin (Sigma, P4333, lot 058M4857V) at 37 °C and 5% CO2.
- The day before plating, place a 15mm round glass/well in 24 well plate and incubate overnight with poly L-ornitine (Sigma, P4957).
- Wash with PBS.
- Plate 2x105 cells/well in 500 μl in a 24 well plate with round glasses.
- Grow cells for 24 h at 37 °C and 5% CO2.
- Transfect cells with 0.2 µg/well of a plasmid expressing human SpCas9-T2A-eGFP under the control of the ubiquitous promoter CMV using the calcium phosphate method. For each well, prepare 30 μl 0.25 M CaCl2, add DNA, add 30 μl HBS, incubate for 3 min at RT, and add the whole mix to the cells.
- Change the media after 4-6 h.
- Grow cells for 72 h at 37 °C and 5% CO2.
- Remove the media and wash once with PBS.
- Add 250 μL of 4% PFA and incubate for 7 min.
- Wash 3 times with PBS.
- Add 250 μL of 0.1 M glycine in PBS to block unreacted aldehydes.
- Remove glycine and add blocking solution (5% goat serum + 1% BSA Serum in 0.3% Triton-X100 in 1 x TBS) for 60 min at RT.
- Dilute primary
- mouse anti-Cas9 antibody (antibodies-online, ABIN5074774, Batch N: 1060814) 1:250 or
- mouse anti-2A peptide antibody (antibodies-online, ABIN5074774, lot A-12) 1:250 in blocking solution.
- Add 250 µL/well of primary antibody solution.
- Cover the plate with aluminum foil and Incubate overnight at 4 °C in the dark in a shaking plate.
- Wash wells 3 times with PBS.
- Dilute secondary goat anti-mouse Cy3 (Invitrogen, A10521) 1:400 and DAPI (0.1 ug/ml) in blocking solution.
- Add 250 µL/well of secondary antibody solution.
- Wash wells 3 times with PBS.
- Place the round glasses in microscope slides.
- Add fluorescence mounting medium (DAKO, S3023, lot 10115314) and cover with coverslips.
- Seal the edges of the coverslips with a clear nail polish.
- Let the nail polish dry and store samples at 4 °C in dark.
- Acquire images with Axiovert 200M with a 40X objective.
- Anmerkungen
The insertion of a 2A peptide between 2 coding sequences leads to the translation of a unique protein which is then spliced in two proteins by the cell machinery. In this process, the 2A peptide stays bound to the C-terminus of the first protein and it can be used as a tag. In this experiment, the T2A peptide is bound to the SaCas9 protein and it can be used as a positive control. Moreover we expect that cells expressing eGFP should express the saCas9 protein as well.
The SaCas9 antibody ABIN6972659 specifically labels the targeted antigen in HEK293 cells transfected with a plasmid encoding SaCas9-T2A-eGFP. Importantly, cells are always colabeled with eGFP and the antibody.
As a control, the T2A antibody shows a similar pattern.
Validierung #104240 (Immunofluorescence)![Erfolgreich validiert 'Independent Validation' Siegel]()
![Erfolgreich validiert 'Independent Validation' Siegel]() Validierungsbilder
Validierungsbilder![HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.]() HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.
Protokoll
HEK-293 cells transfected with a plasmid expressing SpCas9-T2A-eGFP and stained with ABIN4880057 and a Cy3 conjugated anti-mouse antibody (first row), an anti-2A antibody and a Cy3 conjugated anti-mouse antibody (second row). Staining of transfected cells with the secondary antibody alone (third row) or non-transfected cells with ABIN4880057 or the Cy3–conjugated secondary antibody (forth row) or the the 2A-antibody and Cy3 secondary (fifth row) served as negative controls.
Protokoll -
- Format
- Liquid
- Konzentration
- 1 mg/mL
- Buffer
- Purified antibody (from supernatant) containing PBS + 0.09 % sodium azide
- Konservierungsmittel
- Sodium azide
- Vorsichtsmaßnahmen
- This product contains Sodium azide: a POISONOUS AND HAZARDOUS SUBSTANCE which should be handled by trained staff only.
-
- Target
- CRISPR-Cas9
- Andere Bezeichnung
- CRISPR/Cas9
- Gen-ID
- 901176
-

 (2 validations)
(2 validations)




