HOXA13 Antikörper (AA 332-388)
Kurzübersicht für HOXA13 Antikörper (AA 332-388) (ABIN1386749)
Target
Alle HOXA13 Antikörper anzeigenReaktivität
Wirt
Klonalität
Konjugat
Applikation
-
-
Bindungsspezifität
- AA 332-388
-
Kreuzreaktivität
- Human
-
Homologie
- Mouse,Rat,Cow,Sheep,Pig,Horse,Chicken,Rabbit
-
Aufreinigung
- Purified by Protein A.
-
Immunogen
- KLH conjugated synthetic peptide derived from human HOXA13
-
Isotyp
- IgG
-
-
-
-
Applikationshinweise
-
WB 1:300-5000
ELISA 1:500-1000
IHC-P 1:200-400
IHC-F 1:100-500
IF(IHC-P) 1:50-200
IF(IHC-F) 1:50-200
IF(ICC) 1:50-200
ICC 1:100-500
CUT&RUN 1:100 -
Beschränkungen
- Nur für Forschungszwecke einsetzbar
-
-
- by
- Gianluca Zambanini, Anna Nordin and Claudio Cantù; Cantù Lab, Gene Regulation during Development and Disease, Linköping University
- No.
- #104368
- Datum
- 26.04.2023
- Antigen
- HOXA13
- Chargennummer
- AD02263837
- Validierte Anwendung
- Cleavage Under Targets and Release Using Nuclease
- Positivkontrolle
Polyclonal rabbit anti-H3K4me (antibodies-online, ABIN3023251)
- Negativkontrolle
Polyclonal guinea pig anti-rabbit IgG (antibodies-online, ABIN101961)
- Bewertung
- Primärantikörper
- ABIN1386749
- Sekundärantikörper
- Full Protocol
- Cell harvest and nuclear extraction
- Dissect 3 Fore limbs (11.5 DAC) from RjOrl:SWISS embryos for each sample.
- Dissociate the tissue into single cells in TrypLE for 15 min at 37 °C.
- Centrifuge cell solution 5 min at 800 x g at RT.
- Remove the liquid carefully.
- Gently resuspend cells in 1 mL of Nuclear Extraction Buffer (20 mM HEPES-KOH pH 8.2, 20% Glycerol, 0,05% IGEPAL, 0.5 mM Spermidine, 10 mM KCl, Roche Complete Protease Inhibitor EDTA-free).
- Move the solution to a 2 mL centrifuge tube.
- Pellet the nuclei 800 x g for 5 min.
- Repeat the NE wash twice for a total of three washes.
- Resuspend the nuclei in 20 µL NE Buffer per sample.
- Concanavalin A beads preparation
- Prepare one 2 mL microcentrifuge tube.
- Gently resuspend the magnetic Concanavalin A Beads (antibodies-online, ABIN6952467).
- Pipette 20 µL Con A Beads slurry for each sample into the 2 mL microcentrifuge tube.
- Place the tube on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Pipette 1 mL Binding Buffer (20 mM HEPES pH 7.5, 10 mM KCl, 1 mM CaCl2, 1 mM MnCl2) into the tube and resuspend ConA beads by gentle pipetting.
- Spin down the liquid from the lid with a quick pulse in a table-top centrifuge.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Repeat the wash twice for a total of three washes.
- Gently resuspend the ConA Beads in a volume of Binding Buffer corresponding to the original volume of bead slurry, i.e. 20 µL per sample.
- Nuclei immobilization – binding to Concanavalin A beads
- Carefully vortex the nuclei suspension and add 20 µL of the Con A beads in Binding Buffer to the cell suspension for each sample.
- Close tube tightly incubates 10 min at 4 °C.
- Put the 1.5 mL tube on the magnet rack and when the liquid is clear remove the supernatant.
- Resuspend the beads in 1 mL of EDTA Wash Buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM Spermidine, Roche Complete Protease Inhibitor EDTA-free, 2 mM EDTA).
- Incubate for 5 min at RT.
- Place the tube on the magnet stand and when the liquid is clear remove the supernatant.
- Resuspend the beads in 200 µL of Wash Buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM Spermidine, Roche Complete Protease Inhibitor EDTA-free) per sample.
- Primary antibody binding
- Divide nuclei suspension into separate 200 µL PCR tubes, one for each antibody (150,000 cells per sample).
- Add 2 µL antibody (anti-HOXA13 antibody ABIN1386749, anti-H3K4me positive control antibody ABIN3023251, guinea pig anti-rabbit IgG negative control antibody ABIN101961) to the respective tube, corresponding to a 1:100 dilution.
- Incubate ON at 4 °C.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Wash with 200 µL of Wash buffer (to accelerate the process use a multichannel pipette).
- Repeat the wash for a total of five washes.
- pAG-MNase Binding
- Prepare a 1.5 mL microcentrifuge tube containing 200 µL of pAG mix pear sample (200 µL of wash buffer + 120 ng pAG-MNase per sample).
- Place the PCR tubes with the sample on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove tubes from the magnetic stand.
- Resuspend the beads in 200 µL of pAG-MNase premix.
- Incubate for 30 min at 4 °C.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Wash with 200 µL of Wash Buffer using a multichannel pipette to accelerate the process.
- Repeat the wash for a total of five washes.
- Resuspend in 200 µL of Wash Buffer.
- MNase digestion and release of pAG-MNase-antibody-chromatin complexes
- Place PCR tubes on ice and allow to chill.
- Prepare a 1.5 mL microcentrifuge tube with 51 µL of 2 mM CaCl2 mix per sample (50 µL Wash Buffer + 1 µL 100 mM CaCl2) and let it chill on ice.
- Always in ice, place the samples on the magnetic rack and when the liquid is clear remove the supernatant.
- Resuspend the samples in 50 µL of the 2 mM CaCl2 mix and incubate in ice for exactly 30 min.
- Place the sample on the magnet stand and when the liquid is clear move the supernatant in fresh collection tubes with 3 µL of EDTA/EGTA 0.25 M (Digestion buffer).
- Resuspend the sample in 47 µL of 1x Urea STOP Buffer (8.5 M Urea, 100 mM NaCl, 2 mM EGTA, 2 mM EDTA, 0,5% IGEPAL).
- Incubate the samples for 1 h at 4 °C.
- Transfer the supernatant containing the pAG-MNase-bound digested chromatin fragments to the previously collected digestion buffer.
- DNA Clean up
- Take the Mag-Bind® TotalPure NGS beads (Omega Bio-Tek, M1378-01) from the storage and wait until they are RT.
- Add 2x volume of beads to each sample (e.g. 100 µL of beads for 50 µL of sample).
- Incubate the beads and the sample for 15 min at RT.
- During incubation prepare fresh EtOH 80%.
- Place the PCR tubes on a magnet stand and when the liquid is clear remove the supernatant.
- Add 200 µl of fresh 80% EtOH to the sample without disturbing the.
- Incubate 30 sec at RT.
- Remove the EtOH from the sample.
- Repeat the wash with 80% EtOH.
- Resuspend the beads in 25 µL of 10 mM Tris.
- Incubate the sample for 2 min at RT.
- Repeat the 2x beads clean up as described before (this time with 50 µL of beads for each sample).
- Resuspend the beads and DNA in 20 µL of 10 mM Tris.
- Library preparation and sequencing
- Prepare Libraries using KAPA HyperPrep Kit using KAPA Dual-Indexed adapters according to protocol.
- Sequence samples on an Illumina NextSeq 500 sequencer, using a NextSeq 500/550 High Output Kit v2.5 (75 Cycles), 36 bp PE.
- Peak calling
- Trim reads using using bbTools bbduk (BBMap - Bushnell B. - sourceforge.net/projects/bbmap/) to remove adapters, artifacts and repeat sequences.
- Map aligned reads to the mm10 mouse genome using bowtie with options -m 1 -v 0 -I 0 -X 500.
- Use SAMtools to convert SAM files to BAM files and remove duplicates.
- Use BEDtools genomecov to produce Bedgraph files.
- Call peaks using SEACR with a 0.001 threshold and the option norm stringent.
- Anmerkungen
The protocol is published in Zambanini, G. et al. A New CUT&RUN Low Volume-Urea (LoV-U) protocol uncovers Wnt/β-catenin tissue-specific genomic targets. Development (2022). PMID 36355069
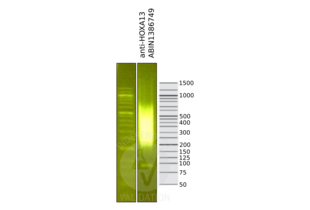
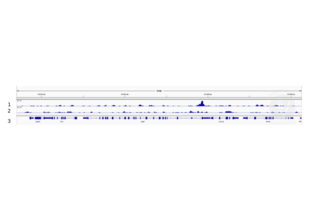
Validierung #104368 (Cleavage Under Targets and Release Using Nuclease)![Erfolgreich validiert 'Independent Validation' Siegel]()
![Erfolgreich validiert 'Independent Validation' Siegel]() ValidierungsbilderProtokoll
ValidierungsbilderProtokoll -
-
Format
- Liquid
-
Konzentration
- 1 μg/μL
-
Buffer
- 0.01M TBS( pH 7.4) with 1 % BSA, 0.02 % Proclin300 and 50 % Glycerol.
-
Konservierungsmittel
- ProClin
-
Vorsichtsmaßnahmen
- This product contains ProClin: a POISONOUS AND HAZARDOUS SUBSTANCE, which should be handled by trained staff only.
-
Lagerung
- 4 °C,-20 °C
-
Informationen zur Lagerung
- Shipped at 4°C. Store at -20°C for one year. Avoid repeated freeze/thaw cycles.
-
Haltbarkeit
- 12 months
-
-
- HOXA13 (Homeobox A13 (HOXA13))
-
Andere Bezeichnung
- HOXA13
-
Hintergrund
-
Synonyms: HOX1, HOX1J, Homeobox protein Hox-A13, Homeobox protein Hox-1J, HOXA13
Background: Sequence-specific, AT-rich binding transcription factor which is part of a developmental regulatory system that provides cells with specific positional identities on the anterior-posterior axis. Sequence-specific transcription factor which is part of a developmental regulatory system that provides cells with specific positional identities on the anterior-posterior axis.
-
Gen-ID
- 3209
-
UniProt
- P31271
Target
-

 (1 Validierung)
(1 Validierung)



