TBX2/3 Antikörper
Kurzübersicht für TBX2/3 Antikörper (ABIN6265491)
Target
Reaktivität
Wirt
Klonalität
Konjugat
Applikation
-
-
Spezifität
- TBX 2/3 Antibody detects endogenous levels of total TBX 2/3.
-
Kreuzreaktivität
- Human, Maus, Ratte
-
Homologie
- Pig(100%), Bovine(100%), Horse(100%), Sheep(100%), Rabbit(100%), Dog(100%), Chicken(100%), Xenopus(100%)
-
Aufreinigung
- The antiserum was purified by peptide affinity chromatography using SulfoLinkTM Coupling Resin (Thermo Fisher Scientific).
-
Isotyp
- IgG
-
-
-
-
Applikationshinweise
- WB: 1:500~1:3000, IF/ICC 1:100-1:500
-
Beschränkungen
- Nur für Forschungszwecke einsetzbar
-
-
- by
- Mattias Pernebrink and Claudio Cantù; Cantù Lab, Gene Regulation during Development and Disease, Linköping University
- No.
- #104234
- Datum
- 01.03.2021
- Antigen
- TBX3
- Chargennummer
- 10H2885
- Validierte Anwendung
- Cleavage Under Targets and Release Using Nuclease
- Positivkontrolle
- Recombinant anti-H3K27me3 CUT&RUN Positive Control antibody (antibodies-online, ABIN6923144)
- Negativkontrolle
- Monoclonal anti-FLAG (Sigma-Aldrich, F3165)
- Bewertung
Passed. ABIN6265491 allows for TBX3 targeted digestion using CUT&RUN.
- Primärantikörper
- ABIN6265491
- Sekundärantikörper
- Full Protocol
- Cell harvest
- Harvest cells from day 10.5 mouse embryo front limbs, estimating 90,000 cells per antibody to be used at RT.
- Centrifuge cell solution 3 min at 600 x g at RT.
- Remove the liquid carefully.
- Gently resuspend cells in 1 mL Wash Buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM Spermidine, Roche Complete Protease Inhibitor EDTA-free) by pipetting and transfer cell solution to a 2 mL microcentrifuge tube.
- Centrifuge cell solution 3 min at 600 x g at RT and discard the supernatant.
- Repeat twice for a total of three washes.
- Resuspend cell pellet in 1 mL Wash Buffer by gently pipetting.
- Concanavalin A beads preparation
- Prepare one 1.5 mL microcentrifuge tube.
- Gently resuspend the magnetic Concanavalin A Beads (antibodies-online, ABIN6923139).
- Pipette 10 µL Con A Beads slurry for each sample into the 1.5 mL microcentrifuge tube.
- Place the tube on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Pipette 1 mL Binding Buffer (20 mM HEPES pH 7.5, 10 mM KCl, 1 mM CaCl2, 1 mM MnCl2) into each tube and resuspend ConA beads by gentle pipetting.
- Spin down the liquid from the lid with a quick pulse in a table-top centrifuge.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Repeat twice for a total of three washes.
- Gently resuspend the ConA Beads in a volume of Binding Buffer corresponding to the original volume of bead slurry, i.e. 10 µL per sample.
- Cell immobilization – binding to Concanavalin A beads
- Carefully vortex the cell suspension and add 10 µL of the Con A beads in Binding Buffer to the cell suspension for each sample.
- Close tube tightly and rotate for 10 min at RT.
- Cell permeabilization and primary antibody binding
- Divide cell suspension into separate 2 mL microcentrifuge tubes, one for each antibody (500,000 cells per sample).
- Place the microcentrifuge tubes on a magnetic stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Place each tube at a low angle on the vortex mixer set to a low speed and add 150 µL Digitonin Wash buffer (wash buffer with 0.025% (wt/vol) Digitonin) supplemented with 2 mM EDTA.
- Gently vortex the microcentrifuge tubes until the beads are resuspended.
- Add 1.5 µL antibody (Anti TBX3 (ABIN6265491), positive control, and negative control) to the respective tube, corresponding to a 1:100 dilution.
- Rotate the microcentrifuge tubes ON at 4 °C.
- Spin down the liquid and place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Resuspend with 1 mL Digitonin Wash Buffer and mix by inversion. If clumping occurs, gently remove the clumps with a 1 ml pipette tip.
- Repeat once for a total of two washes.
- pA-MNase Binding
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Vortex the sample at low speed and add 150 μL pA-MNase solution at 700 ng/mL (1:200 dilution of a 140 µg/mL glycerol stock in Digitonin Wash Buffer) per sample, gently resuspending the beads by pipetting.
- Rotate the microcentrifuge tubes for 1 h at 4 °C.
- Spin down the liquid and place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Resuspend with 1 mL Digitonin Wash Buffer and mix by inversion. If clumping occurs, gently remove the clumps with a 1 mL pipette tip.
- Repeat once for a total of two washes.
- MNase digestion and release of pA-MNase-antibody-chromatin complexes
- Spin down the liquid from the lid with a quick pulse in a table-top centrifuge.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Place each tube at a low angle on the vortex mixer set to a low speed and add 100 μL Digitonin Wash buffer per sample along the side of the tube.
- Place tubes in a heat block, kept on ice, and allow to chill.
- Add 2 μL 0.1 M CaCl2 to each sample.
- Incubate tubes at 0 °C for 30 min.
- Add 100 μL 2xSTOP buffer (340 mM NaCl, 20 mM EDTA, 4 mM EGTA, 0.05% (wt/vol) Digitonin, 100 μg/mL RNAse A, 50 μg/mL Glycogen).
- Incubate tubes at 37 °C for 30 min.
- Place the tubes on a magnet stand until the fluid is clear.
- Transfer the supernatant containing the pA-MNase-bound digested chromatin fragments to fresh 1.5 mL microcentrifuge tubes.
- DNA extraction
- Add 2 µL 10% SDS to a final concentration of 0.1% and 2.5 µL Proteinase K (20 mg/mL) to each supernatant.
- Gently vortex tubes at a low speed of approximately 1,100 rpm.
- Incubate tubes at 50 °C for 1 h.
- Add 200 µL PCI to tube.
- Vortex tubes thoroughly at high speed until the liquid appears milky.
- Centrifuge tubes in a tabletop centrifuge at 16,000 x g at RT for 5 min.
- Carefully transfer to upper aqueous phase to a fresh 1.5 mL microcentrifuge tube containing 2 µL glycogen (diluted 1:10 to 2 mg/mL from the 20 mg/mL stock solution).
- Add 20 µL 3 M NaOAc pH 5.2.
- Add 400 µL 100% ethanol.
- Place tubes for at -20 °C ON.
- Centrifuge tubes in a tabletop centrifuge at 16,000 x g at 4 °C for 5min.
- Remove the liquid carefully with a pipette.
- Wash pellet with 1ml 70% ethanol.
- Centrifuge tubes in a tabletop centrifuge at 16,000 x g at 4 °C for 1 min.
- Remove the liquid carefully with a pipette.
- Air-dry the pellet, then dissolve in 30 µL 1 mM Tris-HCl, 0.1 mM EDTA.
- Library preparation and sequencing
- Libraries were prepared using KAPA HyperPrep Kit using KAPA Dual-Indexed adapters according to protocol.
- Samples were sequenced on an Illumina NextSeq 500 sequencer, using a NextSeq 500/550 High Output Kit v2.5 (75 Cycles), 36bp PE.
- Peak calling
- Reads were mapped to the GRCm38 (mm10) mouse genome using Bowtie2 with options: --local --very-sensitive- local --no-unal --no-mixed --no-discordant.
- Peaks were called using MACS2 with options -f BAMPE --keep-dup all –nomodel.
- Anmerkungen
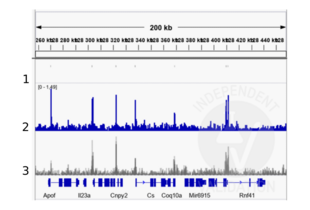
Peaks generated using ABIN6265491 for CUT&RUN aligned well with TBX3 ChIP-seq tracks from the same tissue (Zimmerli et. al., 2020).
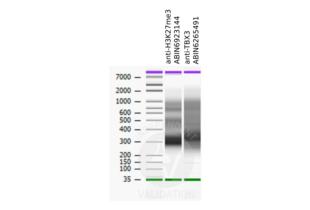
Validierung #104234 (Cleavage Under Targets and Release Using Nuclease)![Erfolgreich validiert 'Independent Validation' Siegel]()
![Erfolgreich validiert 'Independent Validation' Siegel]() Validierungsbilder
Validierungsbilder![Alignment tracks from CUT&RUN targeting TBX3 in mouse front limbs. 1. Peaks called by MACS2 from CUT&RUN data using anti-TBX3 antibody ABIN6265491 in cells derived from mouse embryonal front limbs. 2. Alignment track for CUT&RUN reads obtained using anti-TBX3 antibody ABIN6265491 in mouse embryonal front limbs. 2. Overlayed alignment tracks from ChIP-seq of TBX3 in mouse embryonal front limbs (Zimmerli et. al., 2020). Reads are normalized to sequencing depth per million reads.]() Alignment tracks from CUT&RUN targeting TBX3 in mouse front limbs. 1. Peaks called by MACS2 from CUT&RUN data using anti-TBX3 antibody ABIN6265491 in cells derived from mouse embryonal front limbs. 2. Alignment track for CUT&RUN reads obtained using anti-TBX3 antibody ABIN6265491 in mouse embryonal front limbs. 2. Overlayed alignment tracks from ChIP-seq of TBX3 in mouse embryonal front limbs (Zimmerli et. al., 2020). Reads are normalized to sequencing depth per million reads.
Protokoll
Alignment tracks from CUT&RUN targeting TBX3 in mouse front limbs. 1. Peaks called by MACS2 from CUT&RUN data using anti-TBX3 antibody ABIN6265491 in cells derived from mouse embryonal front limbs. 2. Alignment track for CUT&RUN reads obtained using anti-TBX3 antibody ABIN6265491 in mouse embryonal front limbs. 2. Overlayed alignment tracks from ChIP-seq of TBX3 in mouse embryonal front limbs (Zimmerli et. al., 2020). Reads are normalized to sequencing depth per million reads.
Protokoll -
-
Format
- Liquid
-
Konzentration
- 1 mg/mL
-
Buffer
- Rabbit IgG in phosphate buffered saline , pH 7.4, 150 mM NaCl, 0.02 % sodium azide and 50 % glycerol.
-
Konservierungsmittel
- Sodium azide
-
Vorsichtsmaßnahmen
- This product contains Sodium azide: a POISONOUS AND HAZARDOUS SUBSTANCE which should be handled by trained staff only.
-
Lagerung
- -20 °C
-
Informationen zur Lagerung
- Store at -20 °C.Stable for 12 months from date of receipt
-
Haltbarkeit
- 12 months
-
-
-

 (1 Validierung)
(1 Validierung)



